
Therefore about 27% of all absorption reactions result in radiative capture of incident neutron. The cross-section for radiative capture for thermal neutrons is about 270 barns (for 0.025 eV neutron). Most absorption reactions result in fission reactions, but a part of reactions result in radiative capture forming 240Pu. For fast neutrons, its fission cross-section is on the order of barns. Plutonium 239 is a fissile isotope, and its fission cross-section for thermal neutrons is about 750 barns (for 0.025 eV neutron). (a) List the execution order of these parts from different iterations of the outer loop before fission.

Fission example code#
Use the F H D code in Figure 11.4(b) and enumerate the execution order of the two parts of the outer loop body: (1) the statements before the inner loop and (2) the inner loop. The capture-to-fission ratio is much smaller than the other two major fissile fuels 235U and 239U. Loop fission splits a loop into two loops. About 94% of all absorption reactions result in fission. Therefore about 6% of all absorption reactions result in radiative capture of neutrons. The process involves the division by utilizing the FtsZ protein, including chromosomal replication, chromosomal segregation, and cell splitting. Most of the bacteria reproduce by this process. A group of different organisms, including both prokaryotes and eukaryotes, divide by binary fission. The cross-section for radiative capture for thermal neutrons is about 45 barns (for 0.0253 eV neutron). Examples of organisms that use Binary Fission. Most absorption reactions result in fission reactions, but a minority results in radiative capture forming 234U.

Uranium 233 is a very good fissile isotope, and its fission cross- section for thermal neutrons is about 531 barns (for 0.0253 eV neutron). About 85% of all absorption reactions result in fission. Therefore about 15% of all absorption reactions result in radiative capture of neutrons. The cross-section for radiative capture for thermal neutrons is about 99 barns (for 0.0253 eV neutron). Most absorption reactions result in fission reactions, but a minority results in radiative capture forming 236U. Compounds of boron and cadmium – strong neutron absorbers – are often used.Uranium 235 is a fissile isotope, and its fission cross-section for thermal neutrons is about 585 barns (for 0.0253 eV neutron). Neutron poisons are used in order to lower this reactivity.
Fission example free#
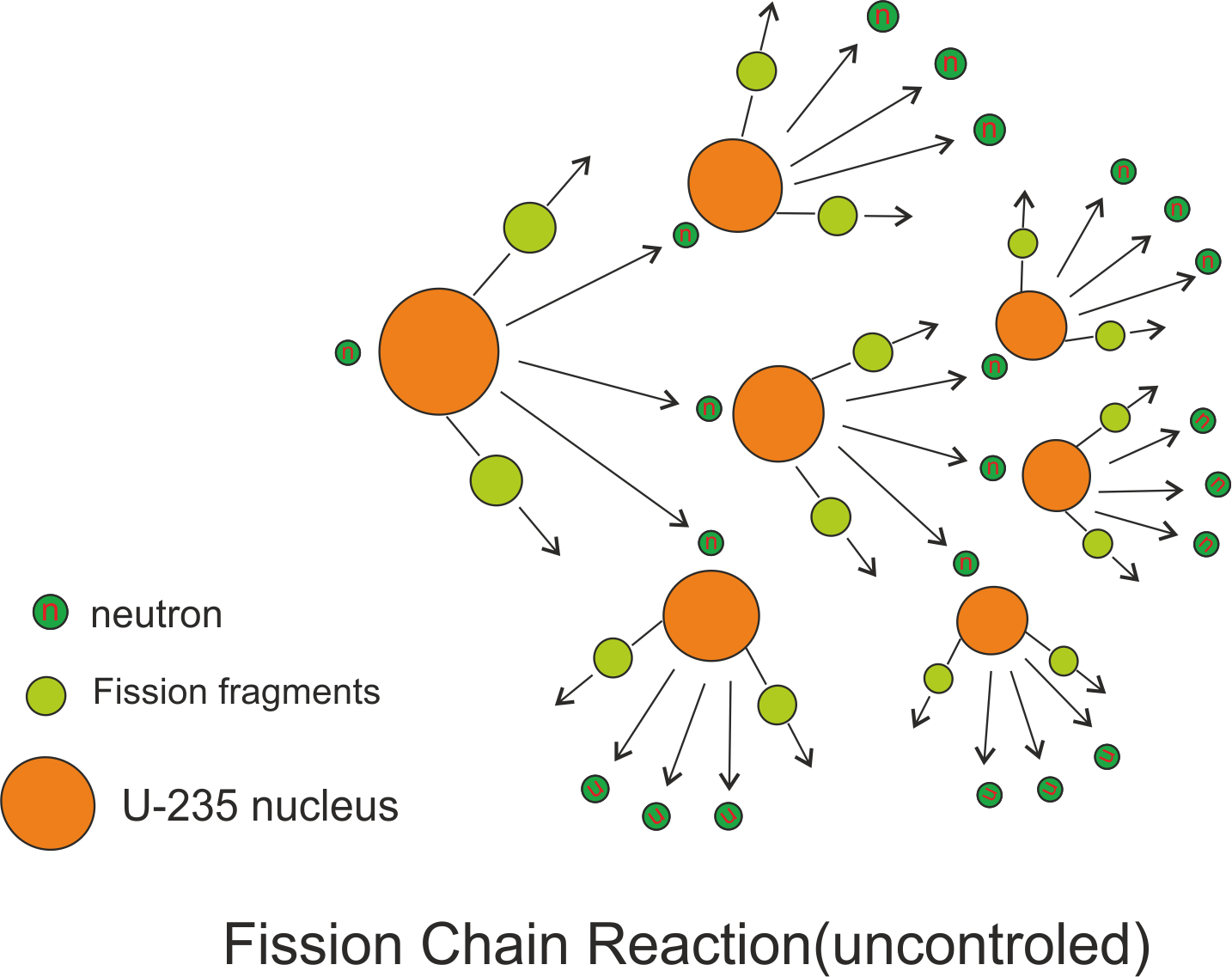
In nuclear reactors, the number of free neutrons must therefore be regulated so that a controlled chain reaction can take place. This reaction would then be continuously repeated at an uncontrolled rate. With no loss of free neutrons, the neutrons released by each fission event would increase and trigger more events, releasing even more neutrons and causing even more fission events. The free neutrons can now induce fission in other nuclei, triggering a chain reaction. This releases 200 megaelectron volts (MeV) of energy. The uranium-236, in turn, splits into fast-moving lighter elements (fission products) – or example, cesium 140 and rubidium 92 – and two or three free neutrons are released (see previous figure). If a thermal (decelerated) neutron is absorbed by a uranium-235 nucleus, it turns briefly into an excited uranium-236 nucleus. Uranium-235 is an example of a fissionable nuclide.


 0 kommentar(er)
0 kommentar(er)
